Diamonds are rare; blue diamonds are even rarer, constituting less than 0.2% of naturally occurring diamonds. Experts say that there are only a handful of notable blue diamonds in the world. And Hope tops that list. This most unique, priceless, brilliant deep blue diamond is currently held most securely at the Smithsonian Museum, Washington DC. More than 350 years ago, John Baptiste Tavernier, a French trader, traveler, fortune hunter held it in his palm and exclaimed at the beautiful violet, but the usual color qualifier has always been Blue.
| Hope Diamond with lighting (Courtesy Wiki) |
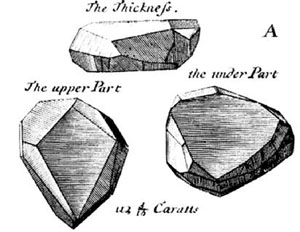 |
| Tavernier's original sketch of French Blue Courtsey: wiki |
During the revengeful loot and plunder of the royal household by the enraged masses, French Blue disappeared along with other crown jewels. Later during the early part of nineteenth century a brilliant blue stone surfaced in the London jewellery market. John Francillon, an English jeweller had his suspicions. There were speculations that this was indeed the French blue. However whosoever owned it in the interim period had it reduced in size to 45.52 ct
and reshaped it - perhaps to escape knowledgeable eyes- before floating it in the market. The blue stone eventually became a part of the English crown jewels collection. In 1830, Henry Philip Hope, a wealthy banker
purchased it from the English King George IV, purportedly as part of a debt settlement. The gem acquired a new, permanent identity The Hope Diamond, a name that didn't change any further with changing ownership. In 1958 Hope Diamond was donated to the Smithsonian Institution, Washington, DC, by the then owner.
So much for the history of Hope. Its chemistry (and also of all natural blue diamonds) is equally interesting. Chemists were fascinated by the brilliant blue and embarked on the job of identifying the cause. In 1971 it was established categorically that traces of boron imparted the blue colour and not aluminium as was believed till then. It was also seen that boron would impart blue colour, only in the absence of other impurities. Hope diamond underwent a systematic, rigorous scientific grading by the Gemological Institute of America (GIA) in 1988. Non-destructive spectroscopic techniques such as Infrared, Ultraviolet and Pulsed luminescence were used to estimate the boron content. Hope recorded a value of 0.6 ppm boron (parts per million). Diamonds are in general insulators, but natural blue diamonds are p-type semi-conductors. Because trivalent boron in a lattice work of tetravalent carbon leaves vacant slots, i.e. holes which facilitate electron jumping.
While chemistry was thus explained, geochemistry still remained elusive for a long time. Because boron is available only in the continental and oceanic crust which run to an average depth of roughly 65 kilometres only. Whereas diamonds are known to be formed at a depth of 100-250 kilometres, in the upper mantle. Several studies alluded to an even deeper zone, a depth of 660 kilometres, for blue diamond formation. So how did boron travel so far down? Perhaps we have an answer now. "Geological pathway for recycling of Earth's surface materials into the mantle are both driven and obscured by palte tectonics" contend Smith et al in the August 2nd issue of Nature Magazine. When continental crust slides over the oceanic crust, lithosphere rich in rocks and minerals gets pushed down through serpentine pathways into the lower mantle.
 |
| Geological process of subduction: Courtsey wikipedia Author: K. D. Schroeder Subduction-en.svg from Wikimedia Commons License:Creative Commons Attribution-ShareAlike 4.0 |
References:
4. Blue boron-bearing diamonds from Earth's lower mantle : Smith et al Nature 560, 84-`87, 2018
No comments:
Post a Comment