Hippocratus (460- 370BC) had alluded to a water channel that encircles the brain. But it took 2000+ years for scientists to put together, piece by piece, a detailed picture highlighting its physiological significance. Thus now we refer to the clear liquid that "waters" the brain and spinal cord as the cerebro-spinal fluid, CSF for short. Specialised ependymal cells in the inner cavity of the brain produce this liquid in pulses. Chemically CSF is very much like serum but with one major difference. The Protein content in serum could be as high as 7000mg/dL, whereas CSF registers only about 35mg/dL. We also know that CSF fulfils multiple responsibilities in the brain, such as cushioning the brain, preserving its buoyancy, supplying nutrients and scavenging waste. The complex network of channels through which CSF flows, together with its associated glial (neuronal) cells, is collectively known as the Glymphatic System. As recent as in in 2015, scientists spotted lymphatic vessels in the meninges too. Meninges is the three tiered protective cover that shields the brain and the spinal cord. It is now realised that the menengeal lymphatic system closely collaborates with the glymphatic system in waste removal from the brain.
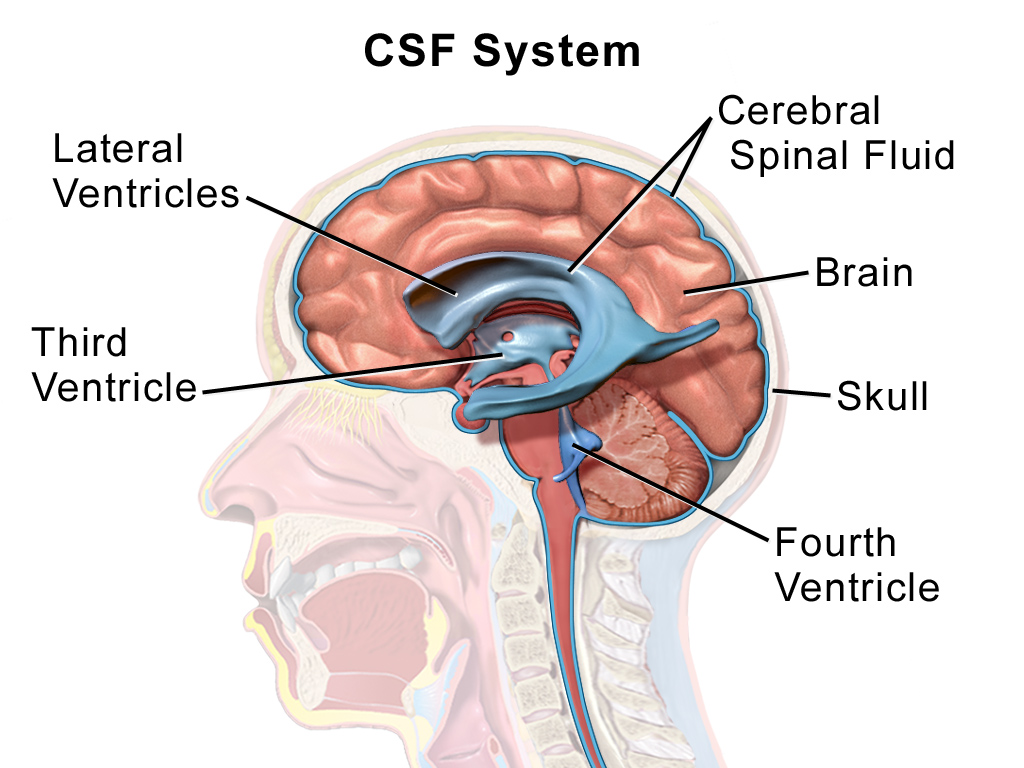 |
| Courtesy: Wikipedia |
The CSF sweeps up the waste and later gets partly absorbed into the venous circulation and partly drained into the lymphatic system for downstream processing. In young healthy adults, this process is rapid and regular. But with age the process becomes sluggish and waste gets accumulated. A typical case in study is the Alzheimer's disease, in which amyloid plaques accumulate. This debris in turn interferes with and impairs neuronal function and also clogs the drainage pathway. Of course it could as well be a combination of over-production of amyloid plaques and under performance of the clearance system.
Scientists were indeed astonished to find that the glymphatic system is mostly dormant when we are awake and gets into fully active mode only when we are asleep. Using sophisticated imaging techniques, it has been demonstrated that the rate of waste clearance from the brain increases by about 60% during the sleep cycle. Hence the extrapolation by Xie et al that the restorative function of sleep may be a consequence of the enhanced removal of potentially neurotoxic waste products that accumulate in the awake central nervous system. Scientists are exploring the possibility of clearing the choked pathways as a novel approach for managing neurodegenerative diseases such as Alzheimer's.
Tailpiece:
Tailpiece:
It has been found that healthy bones facilitate the production of osteocalcin, a hormone necessary for memory retention. Prof.Eric Kandel, who received Nobel Prize in 2000 for unravelling the neurological pathways of learning and memory, has this to say: "If you walk two or three miles a day, you will release sufficient osteocalcin from your bones to combat non-Alzheimer's, age-related memory loss"
REFERENCES:
5. Sleep drives Metabolite clearance from the adult brain
6. Iconic Neuroscientist Eric Kandel Shares This Advice for Combating Memory Loss
6. Iconic Neuroscientist Eric Kandel Shares This Advice for Combating Memory Loss